Medconn Diagnostics Secures Historic FDA Clearance for MQ-3000/MQ3000PT
2025-06-26Shanghai, China – 24/06/2025 – Medconn Diagnostics, a leading innovator in the field of in vitro diagnostics (IVD), is proud to announce that its flagship product, the MQ-3000/MQ3000PT Glycated Hemoglobin Analyzer, has been officially cleared by the U.S. Food and Drug Administration (FDA 510(k)). This marks a significant milestone as Medconn becomes the first Chinese manufacturer to receive FDA clearance for an HPLC-based HbA1c analyzer—a landmark achievement in China's diagnostic industry.
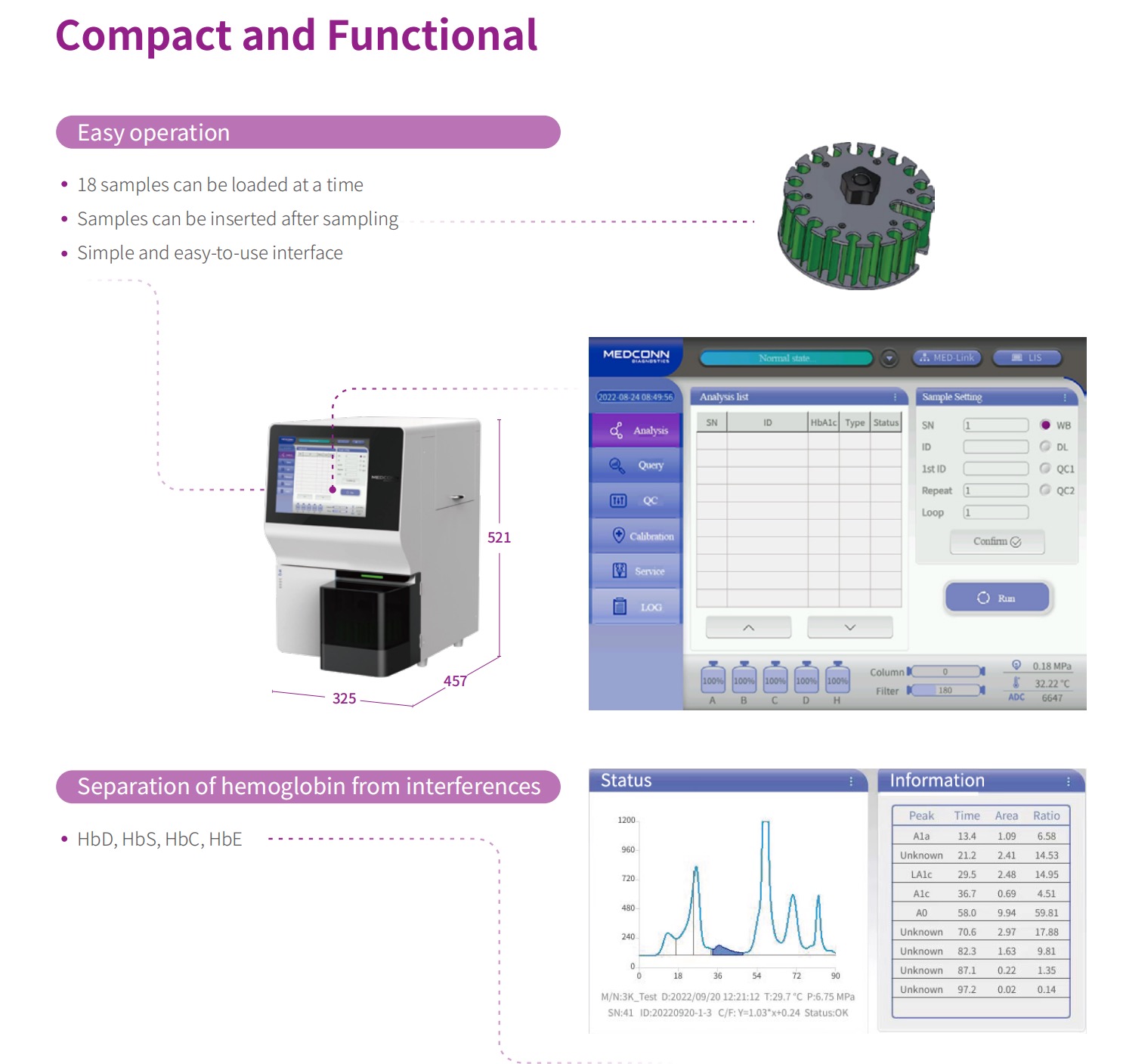
 The MQ Series is based on High-Performance Liquid Chromatography (HPLC), the gold standard method for HbA1c measurement, offering outstanding precision and reliability. The MQ-3000 model is specifically designed to meet the clinical needs of diabetes monitoring and hemoglobin variant detection. It supports rapid analysis, delivering accurate HbA1c results and common variant separation (HbC, HbD, HbS, HbE) within just 115 s/t.
The MQ Series is based on High-Performance Liquid Chromatography (HPLC), the gold standard method for HbA1c measurement, offering outstanding precision and reliability. The MQ-3000 model is specifically designed to meet the clinical needs of diabetes monitoring and hemoglobin variant detection. It supports rapid analysis, delivering accurate HbA1c results and common variant separation (HbC, HbD, HbS, HbE) within just 115 s/t.
 This regulatory milestone signals Medconn’s readiness to expand internationally and provide cutting-edge diagnostic solutions to support the fight against diabetes and hemoglobin disorders.
This regulatory milestone signals Medconn’s readiness to expand internationally and provide cutting-edge diagnostic solutions to support the fight against diabetes and hemoglobin disorders.
About Medconn Diagnostics
Medconn Diagnostics, established in 1992, is a leading company specializing in the research and development, manufacturing, sales, and service of in vitro diagnostic (IVD) products. Headquartered in Hangzhou, China, Medconn Diagnostics serves a broad market across most provinces and regions in China, offering a comprehensive range of products, including HbA1c (HPLC) testing, Hematology, CLIA, Cardiovascular tests , Biochemistry, etc.